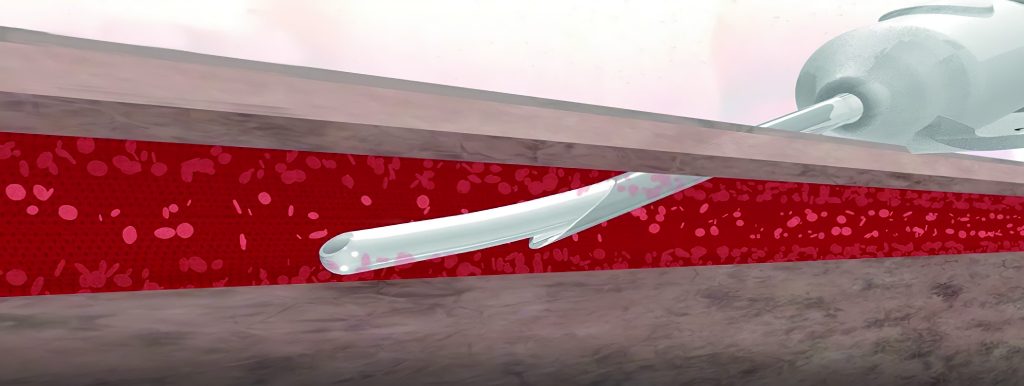
The latest technology from SkyDance Vascular is designed to improve safety measures for the use of Peripheral Intravenous Catheters (PIVC)
DELRAY BEACH, Florida. (April 18, 2023) – Today, SkyDance Vascular, Inc. (SkyDance) announced the U.S. Food and Drug Administration (FDA) 510(k) clearance of the Osprey Peripheral IV Catheter System (OspreyIV), a revolution in peripheral vascular access. The Company also announced additional patent awards from the United States Patent and Trademark Office (USPTO), strengthening the Company’s claim that its products are designed to reduce the number of known complications associated with the traditional PIVC’s.
“What excites me the most about the OspreyIV is the innovation it brings to the healthcare system and that it is a great example of how modernizing a simple medical device can drive efficiencies and reduce healthcare risks,” said Bill Bold, Chief Executive Officer of SkyDance. “With FDA clearance and healthcare systems being forced to focus on safer environments, increased patient satisfaction, and lower costs, the time is right for the OspreyIV to fulfill these needs.”
The OspreyIV was designed to eliminate catheter exposure to infection-causing skin bacteria, maintain needle stick safety during use, and reduce the risk of complications that arise from the regular use of current PIVC technology. The OspreyIV is the only protected catheter delivery system that utilizes Skin Avoidance Technology, creating a physical barrier between the skin and the catheter by housing the catheter inside of the needle.
“The through-the-needle design will be a game changer for inserters of IV catheters,” said Mary Smith, RN, VA-BC, President of CVC Health Care LLC. “Decreasing trauma and successfully inserting devices on the first attempt will prevent harm to the patient and improve the patient’s experience with this procedure. I am excited to share this new product with my colleagues in vascular access!”
This unique design is intended to contribute to minimizing the potential risk of infection while also reducing the potential of other catheter complications, such as phlebitis, infiltration, and extravasation.
“Building on the company’s first-in-class medical technologies, SkyDance is committed to continuously expanding our innovative solutions,” said Mike Anstett, RN, VA-BC, SkyDance’s Founder, announced, “Our investment in through-the-needle peripheral vascular access technologies will not only reduce the risk for patients but work to lower the cost of extended treatments that often arise with current solutions.”
The Osprey IV delivery system is shipping now in limited supplies.
About SkyDance Vascular
SkyDance Vascular, founded in 2017, has redesigned the Peripheral Intravenous Catheter (PIVC). The new product, called the OspreyIV is expected to positively impact insertion-related catheter contamination by utilizing a uniquely designed process called Skin Avoidance Technology. Its goal will be to deliver greater first-attempt success and lower complication rates, improve dwell times, greater completion of therapy rates, and improve patient satisfaction. The company has assembled an executive leadership group comprised of individuals with decades of executive, clinical, regulatory, and engineering experience, and who together have built and successfully exited other companies in the vascular access space. For more information, visit www.skydancevascular.com or contact Bill Bold at bill.bold@ospreyvascular.com.
About Mary Smith RN, VA-BC, President
Mary has been a leading voice in Vascular Access education for over 20 years. She started and managed a Vascular Access Team at her local hospital in Wisconsin that went 14 years without a CLABSI. She founded CVC Healthcare in 2012 after several years of observing poor practices and identifying the need to educate all who insert and care for devices. She speaks locally, regionally, and internationally on topics related to best practices. CVC Health Care has become the largest independent provider of Vascular Access education in the world.
About SkyDance Vascular
SkyDance Vascular, founded in 2017, has redesigned the Peripheral Intravenous Catheter (PIVC). The new product, called the OspreyIV is expected to positively impact insertion-related catheter contamination by utilizing a uniquely designed process called Skin Avoidance Technology. Its goal will be to deliver greater first-attempt success and lower complication rates, improve dwell times, greater completion of therapy rates, and improve patient satisfaction. The company has assembled an executive leadership group comprised of individuals with decades of executive, clinical, regulatory, and engineering experience who have built and successfully exited other companies in the vascular access space. For more information, visit www.skydancevascular.com or contact Bill Bold at bill.bold@ospreyvascular.com.
About Mary Smith RN, VA-BC, President
Mary has been a leading voice in Vascular Access education for over 20 years. She started and managed a Vascular Access Team at her local hospital in Wisconsin that went 14 years without a CLABSI. She founded CVC Healthcare in 2012 after several years of observing poor practices and identifying the need to educate all who insert and care for devices. She speaks locally, regionally, and internationally on topics related to best practices. CVC Health Care has become the largest independent provider of Vascular Access education in the world.
###