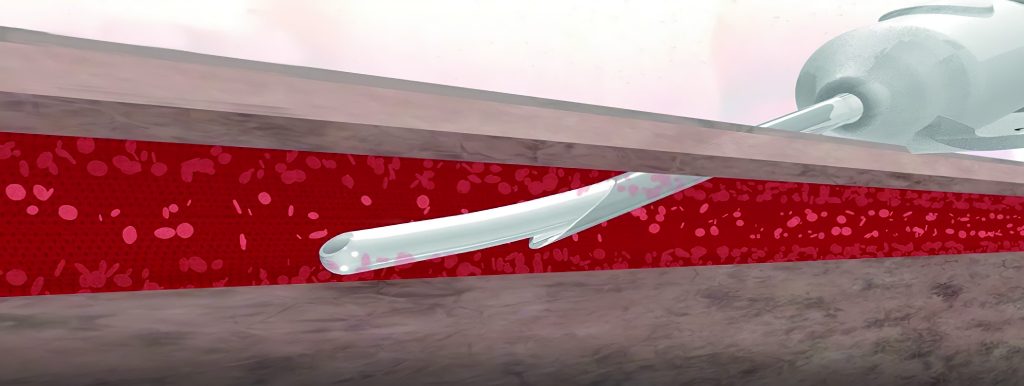
New patent provides additional safety measures for the use of Peripheral Intravenous Catheters (PIV)
DELRAY BEACH, Florida (November 16, 2021) – SkyDance Vascular (“SkyDance” or “the Company”), a newly formed medical device company dedicated to reimagining the Peripheral Intravenous Catheter (PIV), today announced that the United States Patent and Trademark Office (USPTO) has issued a Notice of Allowance for SkyDance’s patent application No. 17/244,898, strengthening the Company’s claim that its products are designed to reduce the number of known complications associated with the traditional PIV’s.
The patent, entitled “Retractable Needle Catheter Delivery Apparatus”, will have 13 claims covering aspects of the Company’s proprietary product that was designed to reduce contamination, improve safety, and extend catheter dwell times.
“This intellectual property approval is an important milestone that provides significant differentiation from any existing or emerging competitive product aimed at improving catheter safety,” said Bill Bold, CEO. “This represents the strategic foundation underpinning our product roadmap and provides important validation for our Osprey platform. We are very excited to extend our unique and patent-protected technology to patients.”
Mike Anstett, RN, VA-BC, Co-Founder and Chief Clinical Officer, commented, “This patent allowance by the USPTO is a significant milestone in the evolution of the Osprey catheter. And, along with our recent clinical validations, SkyDance is poised to create an entirely new category within vascular access.”
Russ Nassof, Executive Vice President of RiskNomics, LLC commented, “Osprey peripheral vascular access catheter appears to reduce the opportunity for adverse events resulting from potential infection, vessel trauma, and catheter failure all of which are potential indications of a failure to meet the evidence-based standard of care. To the extent that the Osprey vascular access catheter is a viable technology, these improvements may have a significant impact upon the prevention of adverse events with minimal risk, cost, liability, and training. It may result in changing the standard of care and improving patient outcomes.”
The Company is targeting for FDA 510k clearance early next year and expects to commercialize the solution in Q2 2022.
About SkyDance Vascular
SkyDance Vascular, founded in 2017, is working to redesign the Peripheral Intravenous Catheter (PIV). Its new family of products, the Osprey catheter delivery system, will be launched in the first half of 2022 and is expected to provide a positive impact on PIV outcomes by utilizing uniquely designed features such as Skin Avoidance Technology, Contoured Directional Flow Tipping, and Passive Needle Retraction. Its goal is to deliver greater first-attempt success and lower complication rates, improved dwell times, greater completion of therapy rates, and increased patient satisfaction. The company has assembled an executive leadership group comprised of individuals with decades of executive, clinical, regulatory, and engineering experience, and who together have successfully built other companies in the vascular access space. For more information, visit https://www.skydancevascular.comor …
Contact Bill Bold at bill.bold@skydancevascular.com.
LinkedIn: https://www.linkedin.com/company/40779227.
About Russel S. Nassof
Mr. Nassof is an attorney who has been involved in the fields of health risk consulting and clinician education for over 20 years concentrating his work in the areas of infection prevention, and risk/liability minimization for healthcare and environmental risk. He has presented hundreds of seminars and webinars to legal, insurance, loss control, healthcare, and risk management groups throughout the United States, Canada, Europe, and Asia. Mr. Nassof has been involved as a consulting/testifying expert on such diverse events as recent outbreaks of Legionnaires’ Disease, Middle East Respiratory Syndrome (MERS), and Ebola, his passion is focused on managing risk and liability for vascular catheter-associated risk and was the first non-clinician elected to the AVA Board of Directors and also served as a reviewer on the 2016 INS Standards. He has coauthored books on environmental/healthcare issues, holds a patent on a fungal sensing device and recently developed a peripheral catheter matrix addressing clinician best practice issues and liability minimization. Mr. Nassof received his Juris Doctorate and undergraduate degrees from Emory University in Atlanta and currently serves as the AVA National Treasurer.
###
Bill Bold
SkyDance Vascular, Inc.
bill.bold@skydancevascular.com
561-573-5360